[無料ダウンロード! √] homogeneous and heterogeneous definition chemistry 764566-Homogeneous and heterogeneous definition chemistry
The homogenous mixture/solution has only one phase that is solid, liquid and gas Examples for the same are;In this animated lecture, I will teach you about 10 examples of homogeneous mixtures and 10 examples of heterogeneous mixtures, the meaning of homogeneous, t Homogeneous mixtures are uniform, that is, their composition is the same wherever you look at it;

Heterogeneous Mixture Definition Examples Video Lesson Transcript Study Com
Homogeneous and heterogeneous definition chemistry
Homogeneous and heterogeneous definition chemistry-Homogeneous Reaction and Heterogeneous Reaction Homogeneous Reaction Any class of reaction that occurs in a singlephase be that solid, liquid or gaseous phase is said to be a homogeneous reaction Now the homogeneous reaction may occur in a single reaction or multiple reactions can take placeHomogeneous reaction, any of a class of chemical reactions that occur in a single phase (gaseous, liquid, or solid), one of two broad classes of reactions—homogeneous and heterogeneous—based on the physical state of the substances present The most important of homogeneous reactions are the reactions between gases (eg, the combination of common household gas and oxygen to




Homogeneous Mixture Definition Examples Video Lesson Transcript Study Com
Find an answer to your question Homogeneous and heterogeneous definition 1 Log in Join now 1 Log in Join now Ask your question Vijy Vijy Chemistry Secondary School Homogeneous and heterogeneous definition 2A chemist mixed a 70% alcohol solution with a 30% alcohol solution to makeIn this animated lecture, I will teach you the concept of mixture, different types of mixture, homogeneous mixture, heterogeneous mixture, difference between
Homogeneous reactions are chemical reactions in which the reactants and products are in the same phase, while heterogeneous reactions have reactants in two or more phases Reactions that take place on the surface of a catalyst of a different phase are also heterogeneous A reaction between two gases or two miscible liquids is homogeneous Homogeneous means the same type Heterogeneous means diverse types Arrays are homogeneous, since you declare the single type as part of the definition Class data tends to be heterogeneous as you have integers, strings, otherDefinition of homogeneous 1) A substance or material that contains only one kind of compound or one element Homogeneous is Latin for "the same kind" An example of a homogeneous substance would be pure water, which only contains the compound H2O or pure table salt that only contains the compound NaCl
This is the definition of homogeneous along with examples In particular, the use of homogeneous in the context of chemistry is describedThis chemistry video tutorial explains the difference between homogeneous and heterogeneous mixtures within the subtopic of the classification of matter ItChemistry Dictionary Definition of Homogeneous If a substance is not homogeneous, it is said to be heterogeneous Example 1 Chemical elements can be homogeneous Examples of homogeneous elements are nitrogen in a balloon, mercury in a thermometer, or gold in an ingot




Heterogeneous Mixture Definition Examples Video Lesson Transcript Study Com



Mixture
A heterogeneous reaction is a chemical reaction where the reactants are in different phases from each other In a homogeneous reaction, the reactants are in the same phase as one anotherWhile heterogeneous mixtures are uneven, with a composition that varies from one point to another In homogeneous mixtures, there seems to be only one component (solute and solvent), but in heterogeneous, we easily visualize more than two componentsLearn the definition of 'homogeneous chemistry' Check out the pronunciation, synonyms and grammar Browse the use examples 'homogeneous chemistry' in the great English corpus




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
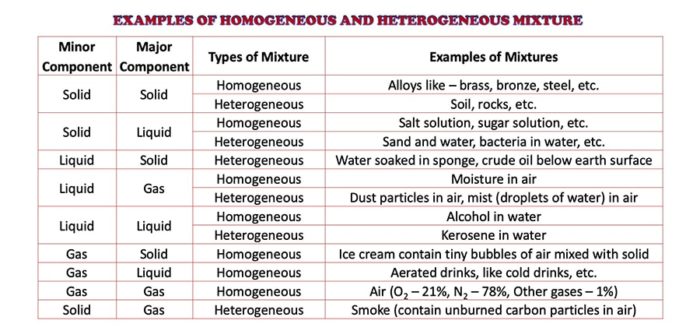



Homogeneous Heterogeneous Mixture Definition Examples Selftution
Yet, there is no similarity in the meaning of homogenous and homogeneous In this Grammarcom article, let us understand some important differences with appropriate examples for each of the words Homogeneous During our chemistry lessons at school, we encountered this word more than often – "two substances having homogeneous characteristicsShare It On Facebook Twitter Email 1 Answer 1 vote answered by Panna01 (472k points) selected by Maisa Best answer Homogenous mixture Homogeneous Mixture Definition Lesson for Kids 3 09 a homogeneous mixture b heterogeneous mixture c element ;




Homogeneous Mixture Definition Examples Tutors Com




Difference Between Homogeneous And Heterogeneous Material Youtube
Moreover, a heterogeneous equilibrium example is also provided in order to learn about the difference between homogeneous and heterogeneous equilibrium The example is given below H 2 O (s) ⇌ H 2 O (l) Homogeneous Equilibrium Example A homogeneous equilibrium can further be divided into two categories There are two main groups of catalysts, heterogeneous and homogeneous In this lesson, we'll learn how they are different, how each type reacts, and look at examples of each Updated Surface organometallic chemistry is an area of heterogeneous catalysis which has recently emerged as a result of a comparative analysis of homogeneous and heterogeneous catalysis The chemical industry has often favored heterogeneous catalysis, but the development of better catalysts has been hindered by the presence of numerous kinds of active sites and also by



Homogeneous Heterogeneous Mixture Definition Examples Selftution




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
In this animated lecture, I will teach you about 10 examples of homogeneous mixtures and 10 examples of heterogeneous mixtures, the meaning of homogeneous, the meaning of heterogeneous, definition of heterogeneous mixture, definition of homogeneous mixture, daily life examples of homogeneous and heterogeneous mixtures, examples of homogeneous andBy definition, a pure substance or a homogeneous mixture consists of a single phase A heterogeneous mixture consists of two or more phases When oil and water are combined, they do not mix evenly, but instead form two separate layers Each of the layers is called a phase In physical chemistry and materials science, the definition of a heterogeneous mixture is somewhat different Here, a homogeneous mixture is one in which all components are in a single phase, while a heterogeneous mixture contains components in different phases Examples of Heterogeneous Mixtures




Heterogeneous Mixture And Homogeneous Mixture Youtube




Difference Between Homogeneous And Heterogeneous Homogeneous Vs Heterogeneous
UNESCO – EOLSS SAMPLE CHAPTERS INORGANIC AND BIOINORGANIC CHEMISTRY – Vol II Homogeneous and Heterogeneous Catalysis Erica Farnetti, Roberta Di Monte and Jan Kašpar ©Encyclopedia of Life Support Systems (EOLSS) terms ab,, and cd,, represent the stoichiometric coefficients of the reactionFor such a reaction we can define the reaction rate asHomogenous and heterogenous mixture chemistry homogeneous mixture and heterogeneous mixture by brajesh mishra introduction to chemistry difference betIn mineral Definition By its definition as a homogeneous solid, a mineral is composed of a single solid substance of uniform composition that cannot be physically separated into simpler chemical compounds Homogeneity is determined relative to the scale on which it is defined A specimen that appears homogeneous to the unaided eye, for




Heterogeneous Equilibrium Homgeneous Equilibrium Concept Chemistry Video By Brightstorm




Mixtures And Solutions Cpd Rsc Education
A heterogeneous mixture is a type of mixture that allows the components to be seen as two or more phases are present A mixture is an example of water Water is a homogeneous mixture of nitrogen, oxygen and smaller amounts of other compounds in the gaseous materials Stay tuned with BYJU'S to learn more interesting topics in ChemistryWhile the term "heterogeneous mixture" sounds like it could be a complicated concept, in reality, it is actually quite simple A mixture is made up of two or more substances, and in a heterogeneous mixture, those substances are not uniformly distributed, meaning that the substances that make up the mixture can be distinguished from one another upon examinationStart studying Chemistry homogeneous or heterogeneous Learn vocabulary, terms, and more with flashcards, games, and other study tools




Chemistry Worksheet




Homogeneous Mixture Definition Examples Video Lesson Transcript Study Com
The degree of heterogeneity (the opposite of homogeneity) is the determining factor of @S@ Source PAC, 1990, 62 , 1193 ( Nomenclature for sampling in analytical chemistry (Recommendations 1990) ) on page 11 Terms Paper The word heterogeneous is an adjective that means composed of different constituents or dissimilar components In chemistry, the word is most often applied to a heterogeneous mixture This is one which has a nonuniform composition A mixture of sand and water is heterogeneousHomogeneous catalysis takes place when the catalyst and the other reactants are all dissolved in the same solution Heterogeneous catalysis typically involves the use of a catalyst that is insoluble, or perhaps only weakly soluble, in the solution in which the reaction takes place




Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Exam Heterogeneous Mixture Chemistry Basics Biology Facts



Is There Any Difference Between Homogeneous Mixture And Solution Here On Quora Previous Answers Are Vague About This While All My Textbooks And Google Sites Say They Are Exactly Same Quora
Department of Chemistry and Nano Science, Ewha Womans University, Seoul, 1750 Korea Faculty of Science and Engineering, Meijo University, Homogeneous and Heterogeneous Photocatalytic Water Oxidation by Persulfate Prof Dr Shunichi Fukuzumi, Corresponding Author fukuzumi@chemengosakauacjp;Definition of Homogeneous and Heterogeneous MixturesBackground Music CreditAlan Walker Fade NCS ReleaseVideo link https//youtube/bM7SZ5SBzyY



1



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples
Solid homogeneous mixture brass is an alloy that is made from metal copper (Cu) and zinc (Zn) Liquid homogeneous mixture a saline solution that is the mixture of water and salt Gas homogenous mixture air is a mixture of different gases such as oxygen, Homogenous Mixture Definition A homogenous mixture is the type of mixture in which the composition of the solute is uniform throughout the mixture Physical Chemistry s Heterogeneous Mixture, Homogenous Mixture, Mixture Post navigation Hard water vs Soft water Definition, 9 Key Differences, ExamplesHeterogeneous definition, different in kind;



1




What Is A Homogeneous Mixture Definition And Examples
The coppercatalyzed azide/alkyne cycloaddition reaction (CuAAC) has emerged as the most useful "click" chemistry Polymer science has profited enormously from CuAAC by its simplicity, ease, scope, applicability and efficiency Basic principles of the CuAAC are reviewed with a focus on homogeneous a The key difference between homogeneous and heterogeneous reactions is that the reactants and products that take part in homogeneous reactions are in the same phase whereas the reactants and products in heterogeneous reactions are in different phases The homogeneity and heterogeneity are two chemical concepts that we describe regarding the uniformity of aA homogeneous thermodynamic system is defined as the one whose chemical composition and physical properties are the same in all parts of the system, or change continuously from one point to another A homogeneous system can be exemplified by imagi
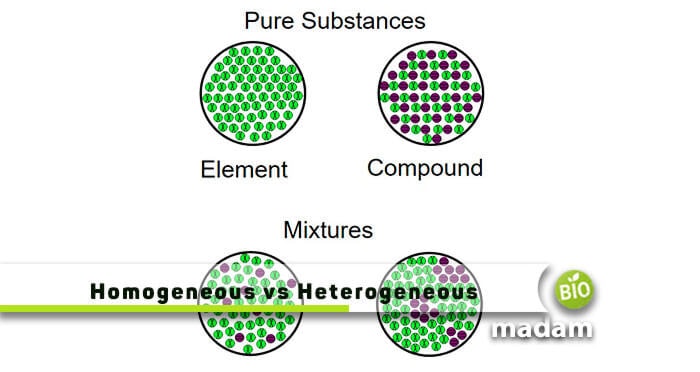



Difference Between Homogeneous Heterogeneous Mixtures Biomadam




Introduction And What Is A Mixture Types Classification Video Examples
Define homogeneous and heterogeneous some basic concepts of chemistry;1011th Grade Chemistry Mixtures & Basics Mixtures & Basics Conversions Atoms Electrons Periodic Trends & Bonding Equations Stoichiometry Part 2 States of Matter & Gas Laws Acids & Bases Mixtures Homogeneous, Heterogeneous Definition of Chemistry Matter and Scientific Method Physical vs Chemical Indicators Mixtures Homogeneous, HeterogeneousThe word heterogeneous is an adjective that means composed of different constituents or dissimilar components In chemistry, the word is most often applied to a heterogeneous mixture This is one which has a nonuniform composition A mixture of sand and water is heterogenous




Homogenous Definition And Examples Biology Online Dictionary




Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Sorting Cards
'I believe that each one of us has a personal responsibility to our planet And since animals, plants, oceans have no voice of their own, we should speak up for them as well Homogenous Mixtures A homogeneous mixture is a mixture in which the composition is uniform throughout the mixture The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample Often it is easy to confuse a homogeneous mixture with a pure substance because they are bothThe prefix "homos" means "same," while the prefix "hetero" means "different" The root of "geneous" is a Greek word, "genos," meaning a group, type, or stock So, homogeneous means all the same group, and heterogeneous means all different groups together




Heterogeneous Mixture Definition Science Trends




Difference Between Homogeneous Mixture And Heterogeneous Mixture




Homogeneous Mixture Definition Examples Tutors Com




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




10 Examples Of Mixtures



Heterogeneous Mixtures




How To Identify Heterogeneous Homogeneous Mixtures




Definition Of Homogeneous And Heterogenous Systems In This Review Download Scientific Diagram
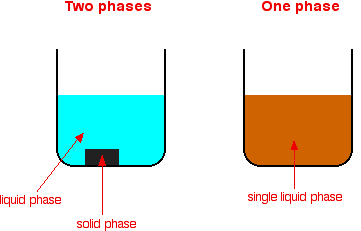



Types Of Catalysis




Mixture Homogeneous And Heterogeneous Mixtures Ck 12 Foundation
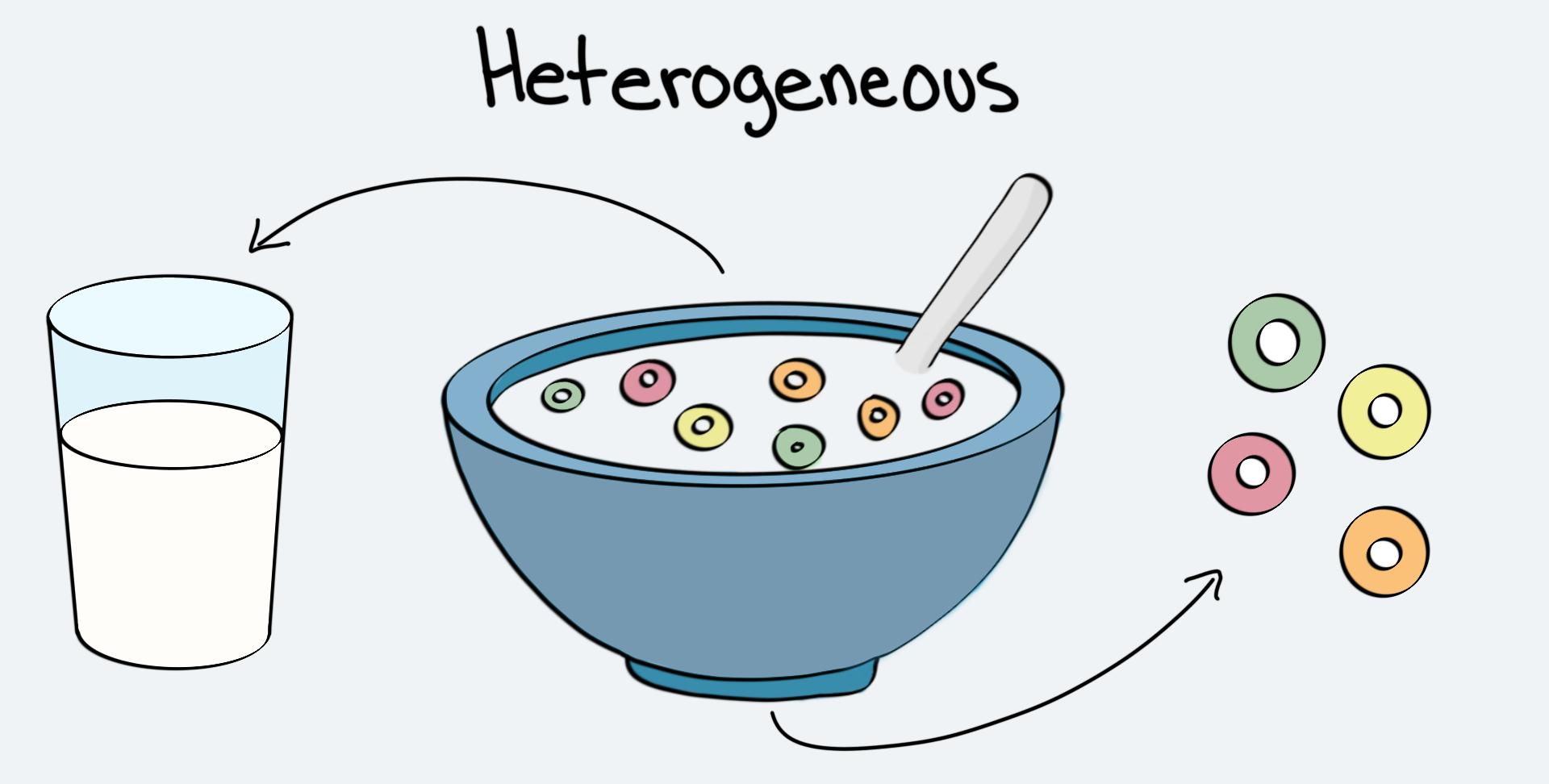



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Homogeneous And Hetrogeneous Mixtures Definition Examples Teachoo
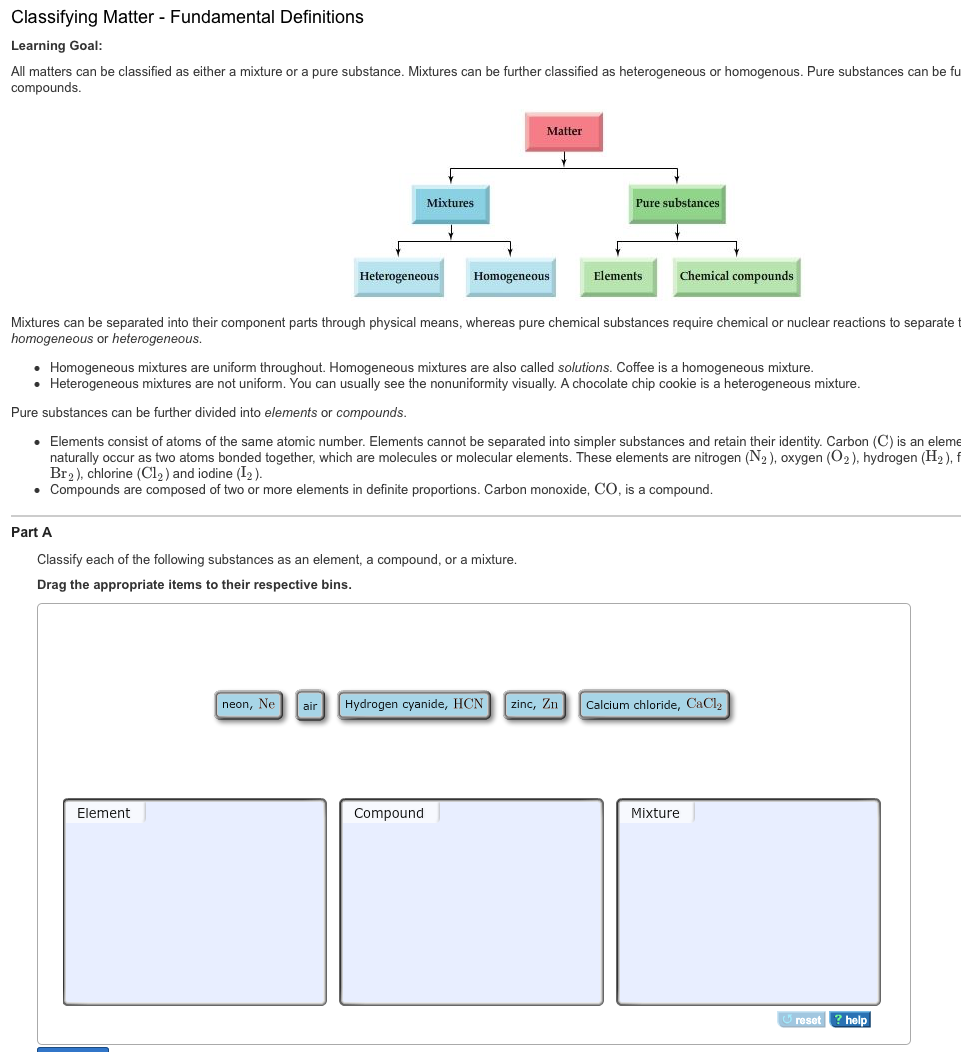



Classifying Matter Fundamental Definitions Chegg Com
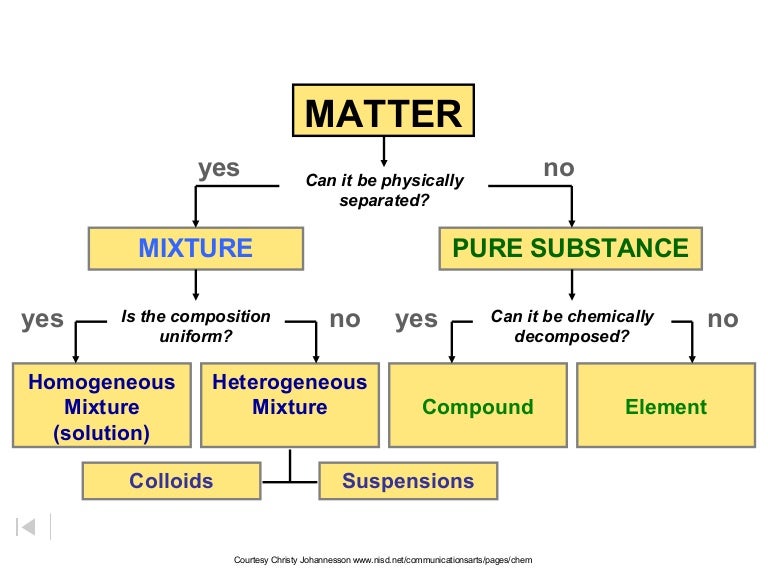



Ch 2 Classification Of Matter Ppt



1



Homogeneous Vs Heterogeneous Energy Education
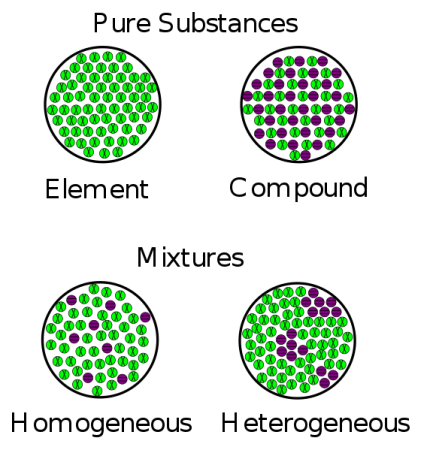



Heterogeneous Mixture Definition Science Trends



Is Sugar A Homogeneous Or Heterogeneous Mixture Quora




Homogeneity And Heterogeneity Wikipedia




5 Examples Of Homogeneous Mixture For Chemistry Class Science Trends



Difference Between Homogeneous And Heterogeneous Welding




Homogenous Definition And Examples Biology Online Dictionary




Chemistry Chapter 3 Matter Properties And Changes 3
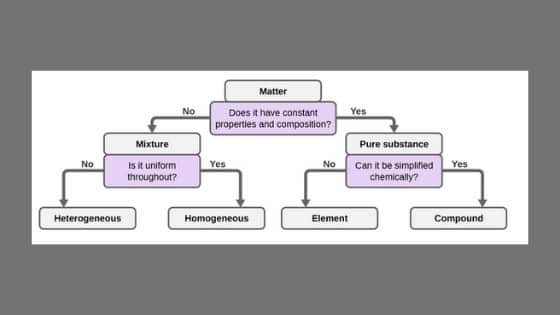



Homogeneous Mixture And Heterogeneous Mixture Ncert Books




Heterogeneous Products Definition Overview Video Lesson Transcript Study Com




Homogeneous Mixture Definition Lesson For Kids Video Lesson Transcript Study Com




Heterogeneous Vs Homogeneous Mixtures



Difference Between Homogeneous And Heterogeneous Mixtures Definition Composition Characteristics Examples




Food Chemistry Distinguish Between Pure Substances And Mixtures Compare Homogeneous And Heterogeneous Mixtures Define Solutions Distinguish Ppt Download




Examples Of Heterogeneous Mixtures Types Made Simple




Q1 Define Homogenous And Heterogeneous Mixture And Chegg Com




Difference Between Homogeneous And Heterogeneous Compare The Difference Between Similar Terms




Heterogeneous Mixture Homogeneous Mixture Worksheet Easy Hard Science




3 4 Classifying Matter According To Its Composition Chemistry Libretexts




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures




What Is A Heterogeneous Mixture Definition And Examples
/definition-of-heterogeneous-mixture-and-examples-605206_final23-ecfa4da6517640429448462eae1f09f7.png)



Definition Of Heterogeneous Mixture With Examples
/GettyImages-548326197-58fe30b63df78ca159cb3f67.jpg)



Heterogeneous Definition Science
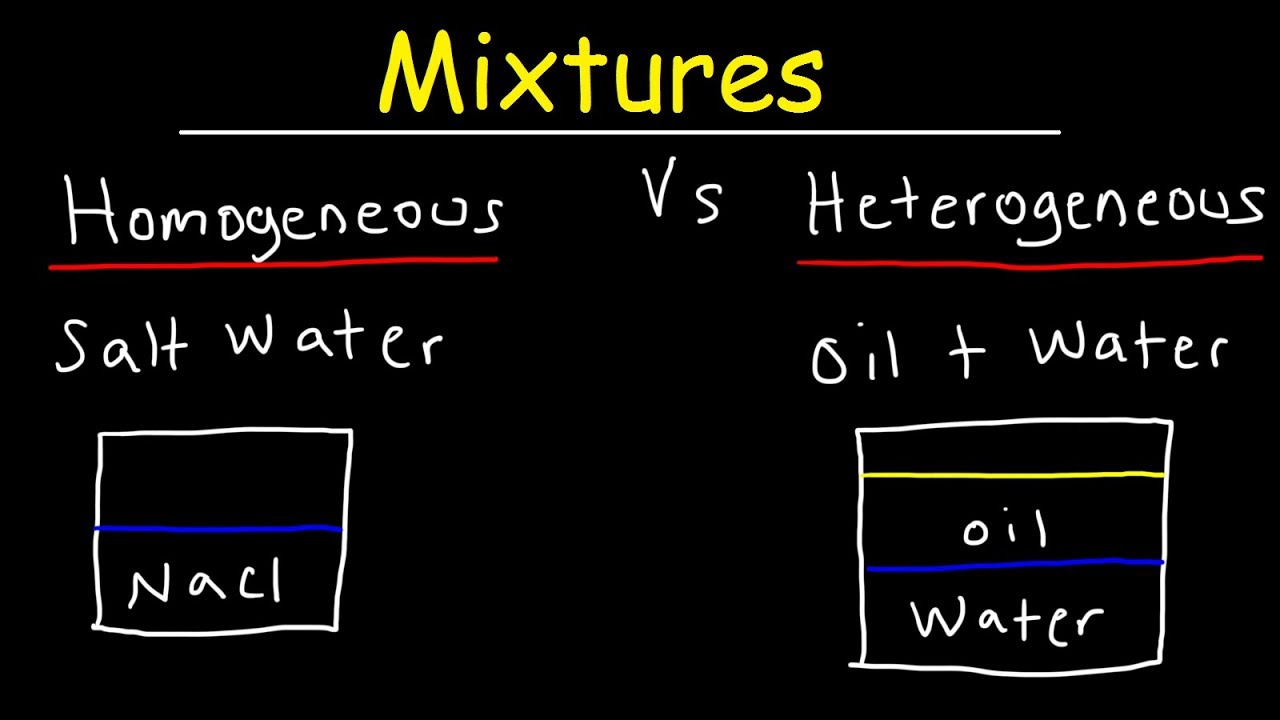



Homogeneous And Heterogeneous Mixtures Examples Classification Of Matter Chemistry Youtube




Homogeneous Mixture And Heterogeneous Mixture Is Matter Around Us Pure Chemistry Class 9 Youtube




Homogeneous Mixture Definition Examples Tutors Com



Elements Compounds And Mixtures Course Hero




Difference Between Homogeneous And Heterogeneous Equilibrium Compare The Difference Between Similar Terms
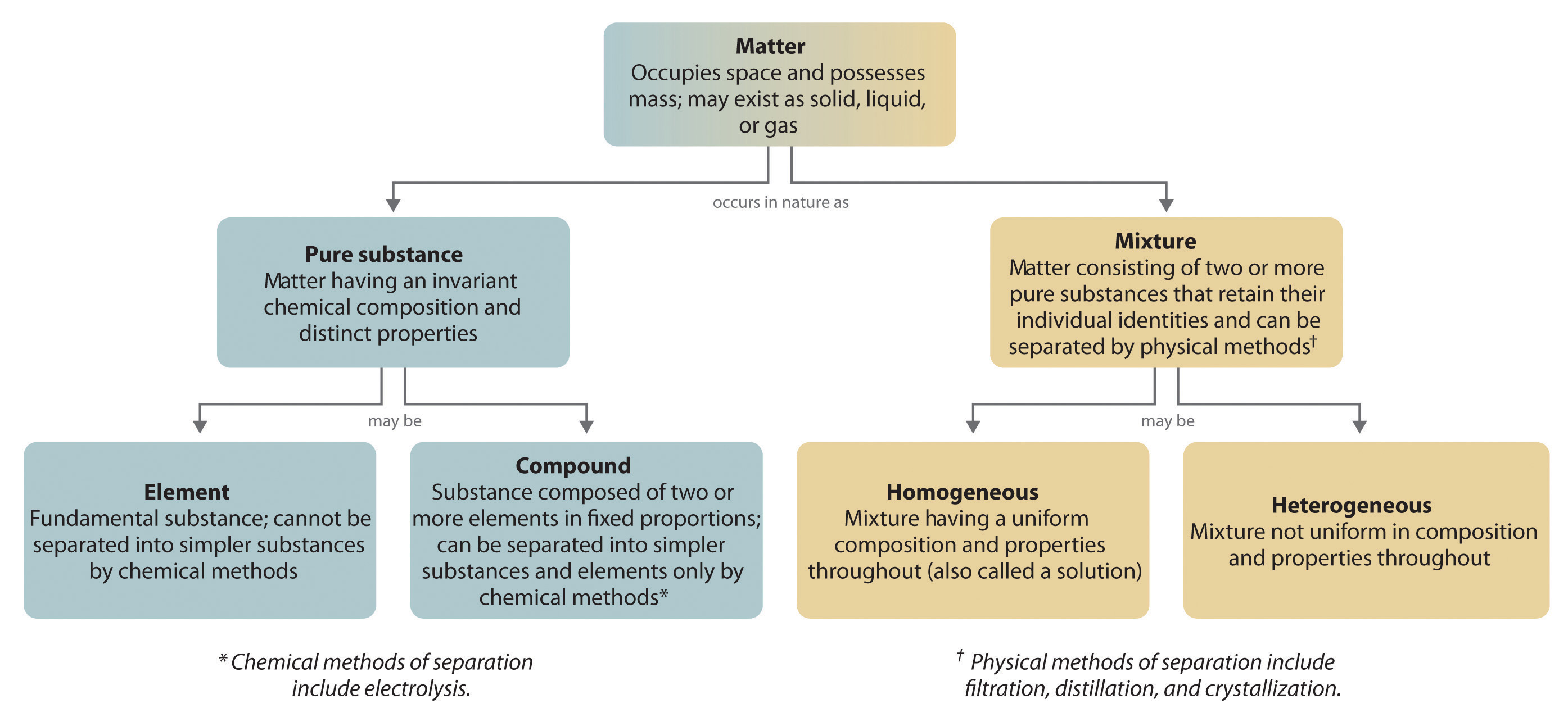



1 3 Classification Of Matter Chemistry Libretexts




Homogeneous And Heterogeneous Mixture Difference Between Homogeneous And Heterogeneous Mixture Youtube




Is Salty Water Homogeneous Or Heterogeneous



1




Heterogeneous And Homogeneous Mixtures In Cooking And Learning Communities By Natalie King And Brandon Connelly Re Writing Chemistry
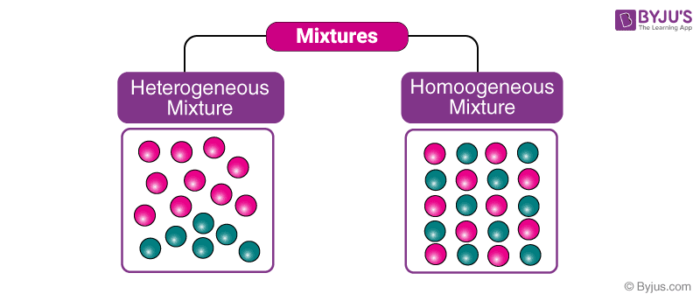



Heterogeneous And Homogeneous Mixture Differences Videos Examples
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures




Heterogeneous And Homogeneous Mixture Differences Videos Examples
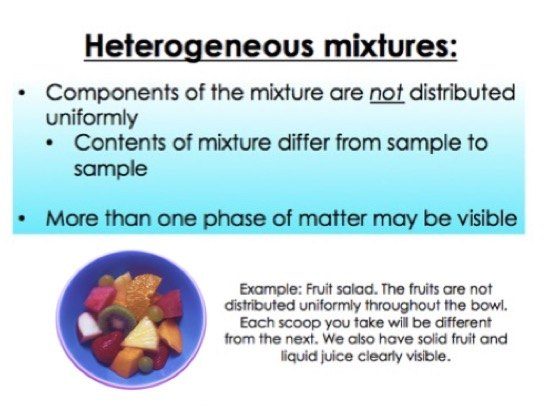



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




Homogenous And Heterogenous Mixtures Chemistry For Non Majors




Homogenous And Heterogenous Mixtures Chemistry For Non Majors




Ways To Separate Mixtures Definition Types Homogeneous Heterogeneous Mixture Eschool



Mixture




Homogeneous Mixture



Homogeneous And Heterogeneous Mixture Nine Science
:max_bytes(150000):strip_icc()/definition-of-pure-substance-605566_FINAL-d1c54ff9183944028aa8e213936affdf.png)



Heterogeneous Vs Homogeneous Mixtures




Examples Of Homogeneous Mixtures Solid Liquid And Gas




Homogeneous Mixture Definition Examples Tutors Com




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures




Homogenous And Heterogenous Mixtures Chemistry For Non Majors




Definition Of Homogeneous And Heterogenous Systems In This Review Download Scientific Diagram




Compound Vs Mixture Difference And Comparison Diffen




Chemistry For Kids Chemical Mixtures



Mixture




Difference Between Homogeneous And Heterogeneous Reactions Compare The Difference Between Similar Terms




Heterogeneous Mixture Lesson For Kids Definition Examples Video Lesson Transcript Study Com



Homogeneous And Heterogeneous Mixtures
/TC_606106-heterogeneous-and-homogeneous-mixtures1-5ac4f1a9642dca0036847e52.png)



Heterogeneous Vs Homogeneous Mixtures




Homogeneous Heterogeneous Mixture Definition Examples Selftution
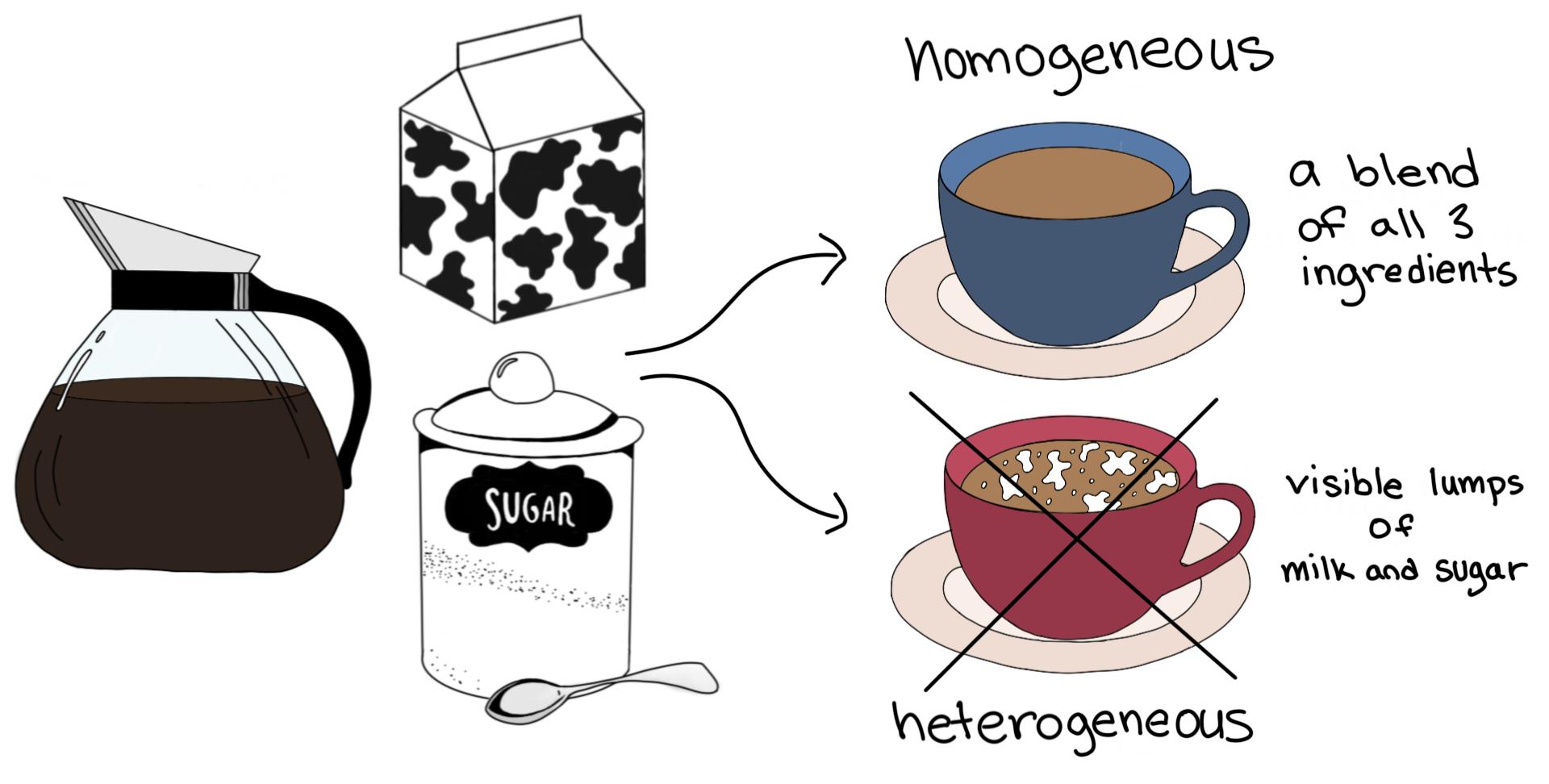



Homogeneous Vs Heterogeneous Mixtures A Comparison Expii




2 1 Matter




How To Identify Heterogeneous Homogeneous Mixtures
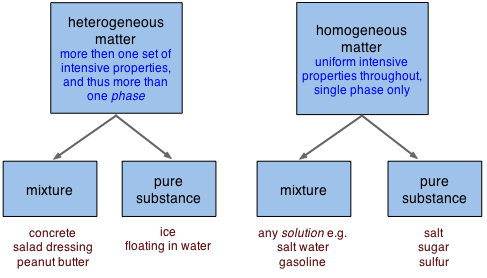



2 1 Classification And Properties Of Matter Chemistry Libretexts




Elements Compounds Mixtures Homogeneous




Chapter 1 Br Section A Br Some Basic Definitions




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
コメント
コメントを投稿